Disclosing Product Information / Appropriate Promotional Activities in Compliance with Laws and Regulations
Our Basic Stance: Operating Policy
In accordance with our Quality Policy, we comply with the Act on Pharmaceuticals and Medical Devices, the Act against Unjustifiable Premiums and Misleading Representations, the Health Promotion Act and other related laws and regulations. With the customer first perspective in mind, we carry out accurate and dignified advertising and marketing activities that do not cause misunderstanding or discomfort, nor do they use false or exaggerated expressions or infringe on human rights.
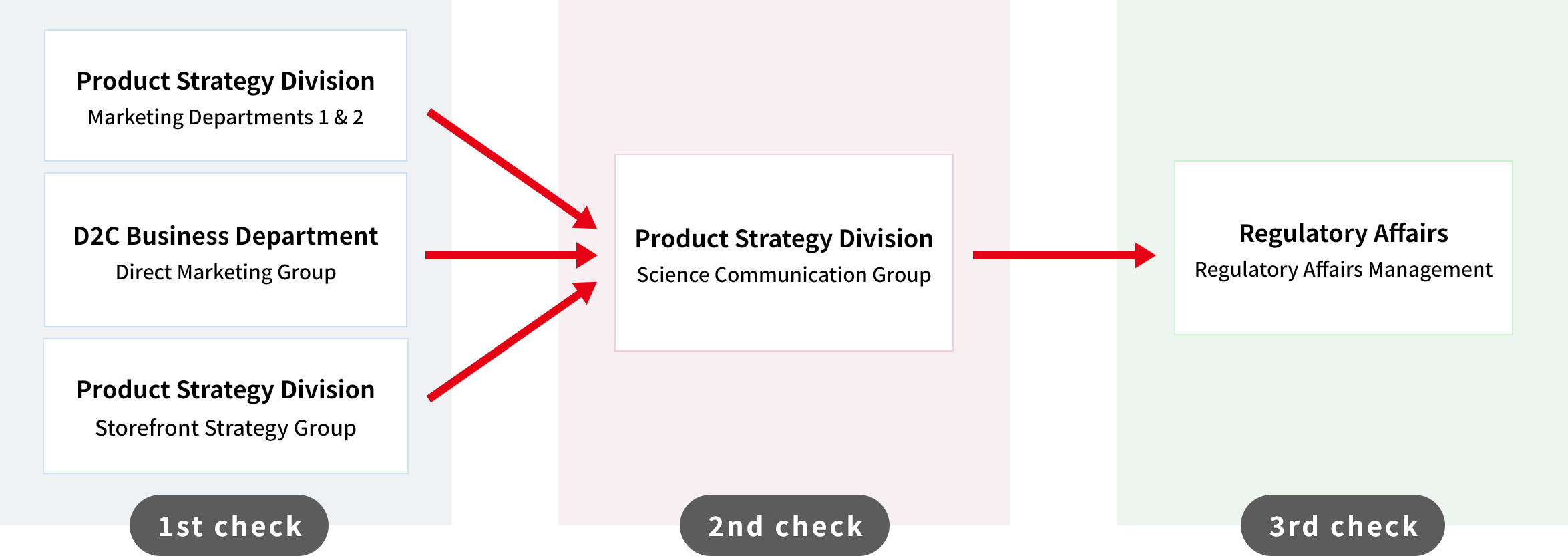
All of the product packaging, accompanying documents, pamphlets, advertisements, and other displays and advertisements that accompany the products we sell to customers are subject to a triple-check system, with the checking department at each step confirming whether there are any deviations from compliance. In order to ensure compliance with the relevant laws and regulations, we have established rules and guidelines for the proper use of advertising, marketing and promotional tools, and we are implementing these in practice. The role of each department and the flow are described below.
Role of each department
| Confirmation step | Main items to be confirmed |
|---|---|
| Originator 1st check |
|
| 2nd check |
|
| 3rd check |
|
| Checks by related departments |
|
Compliance check flow (triple check system)
Staff in each department are participating in seminars run by the Japan Federation of Self-Medication Industries and other external seminars, as well as conducting interviews with relevant parties in an effort to gather information and improve their specialist skills so that the labeling and advertising content reflects the latest legal interpretations, regulatory trends and social trends.
Labeling and Advertising of OTC Drugs and Designated Quasi-Drugs
The information that must be included in the labeling of pharmaceutical products is stipulated in the Act on Pharmaceuticals and Medical Devices With regard to pharmaceutical advertising, there are legal regulations such as the Act on Pharmaceuticals and Medical Devices and the Standards for Fair Advertising Practices concerning Pharmaceuticals, etc., as well as industry-led self-regulation in the form of the Guidelines for the Proper Advertising of Over-the-Counter Medicines issued by the Japan Federation of Self-Medication Industries. We are doing our utmost to ensure appropriate labeling and advertising by complying with the requirements of the Act on Pharmaceuticals and Medical Devices and other regulations governing advertising. Over the past three years (January 2022 to December 2024), we have not had any cases of product labeling or advertising violations that have resulted in us receiving recommendations from regulatory authorities (the Ministry of Health, Labour and Welfare, the Consumer Affairs Agency, the competent consumer organizations, or the Japan Advertising Review Organization (JARO)).
The Japan Federation of Self-Medication Industries has established an Advertising Review Board, which promotes the proper use of advertising, and reviews advertisements for pharmaceuticals and designated quasi-drugs. The Advertising Review Board is made up of third-party external experts and members selected from OTC drug manufacturers. It works to improve the credibility of advertising for OTC drugs and designated quasi-drugs by ensuring that the expressions used in such advertising are appropriate. Alinamin Pharmaceutical participates as a member from the industry side. In this capacity, we are working to ensure that advertising for the OTC drug and designated quasi-drug industries as a whole is appropriate.