Quality Control
Quality Policy
Alinamin Pharmaceutical has established the Quality Policy as follows so that we may fulfill our corporate philosophy of “Innovating GENKI for Tomorrow” and achieve our mission “To support people’s health by providing excellent, high-quality products and services and trustworthy information.”
-
Achieving a stable supply of products of appropriate quality
We will provide a stable supply of products with appropriate quality attributes to satisfy the needs of our customers, including consumers, pharmacies, drugstores, distributors, regulatory authorities (including by complying with approved applications), internal company departments, contracted manufacturers, and affiliated companies.
-
Process (system) assurance
By developing and operating a system that can effectively monitor and manage the operating performance of the manufacturing process and product quality, we will establish and promote a pharmaceutical quality system that assures process capability and enables continuous manufacturing and stable supply of products of adequate quality.
-
Promoting continuous improvement
We will improve our manufacturing processes and reduce quality variations through the reliable implementation of our Quality Policy, the proactive introduction of the latest technologies, quality awareness activities for our manufacturing subcontractors, etc. In addition, we will improve the levels at which we satisfy customer needs and legal requirements, while also consistently fulfilling quality requirements.
Quality Assurance System
System of responsibility and Three Officers structure in Marketing Authorization Holder
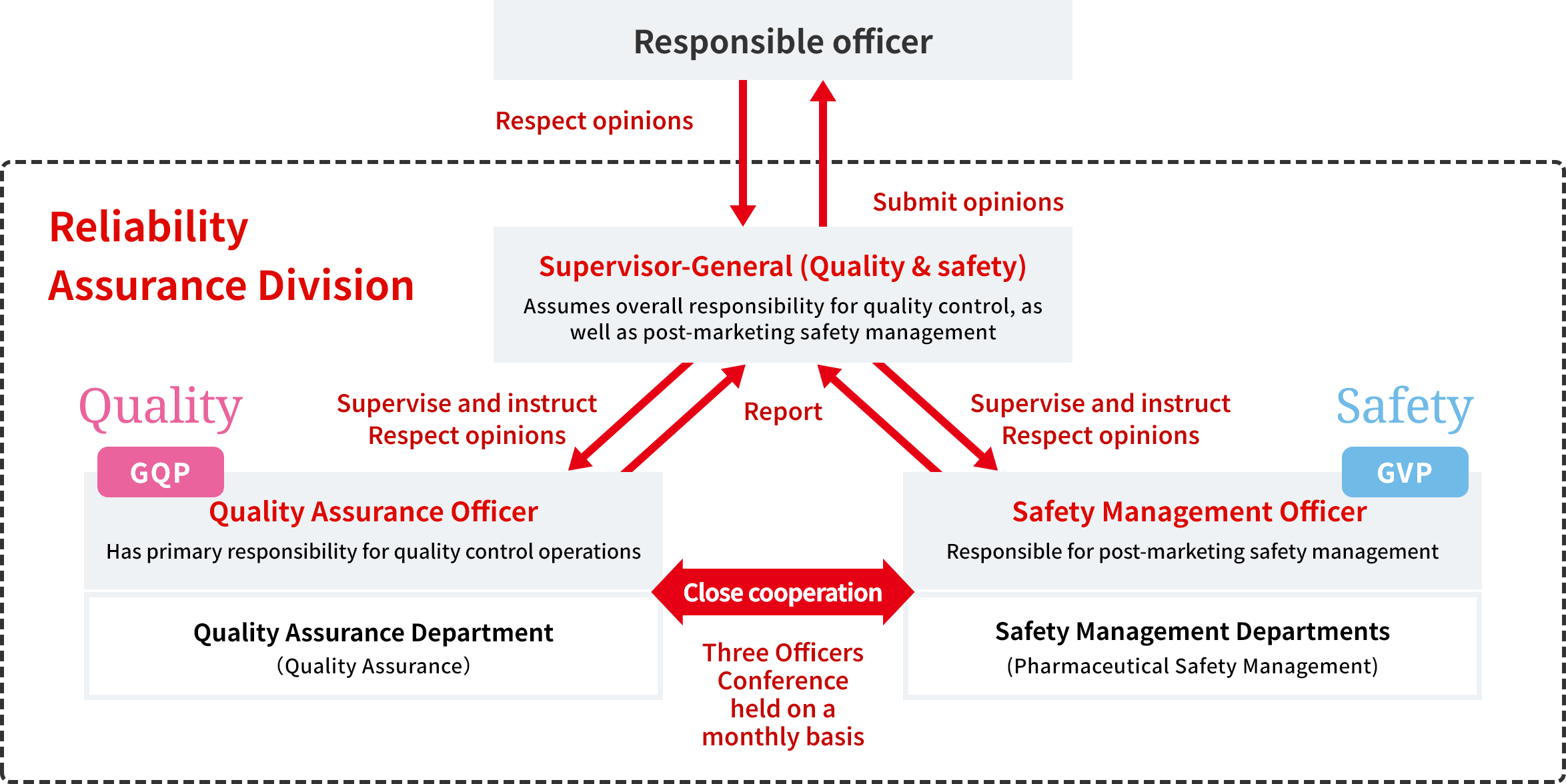
Our quality assurance system consists of a Three Officers Conference held once a month, where the three key parties in Marketing Authorization Holder review and discuss issues. The content of the reviews and discussions at these Conferences are reported to our President, who is the responsible officer, so that issues can be shared. In this way, we are making efforts to improve product quality involving our President.
The purpose of our Three Officers Conference is stipulated in the Company's business management policy, and is to ensure that the Supervisor-General (Quality & Safety) closely coordinates with the Quality Assurance Officer and the Safety Management Officer, while also appropriately supervising the work of Quality Assurance Departments and Safety Management Departments.
The business management policy also defines the structure and authorities of Three Officers as internal control functions. With these standards and organizational arrangements in place, the Supervisor-General (Quality & Safety) makes decisions regarding necessary measures, respecting the opinions of the Quality Assurance Officer and the Safety Management Officer.
Involvement in the product life cycle
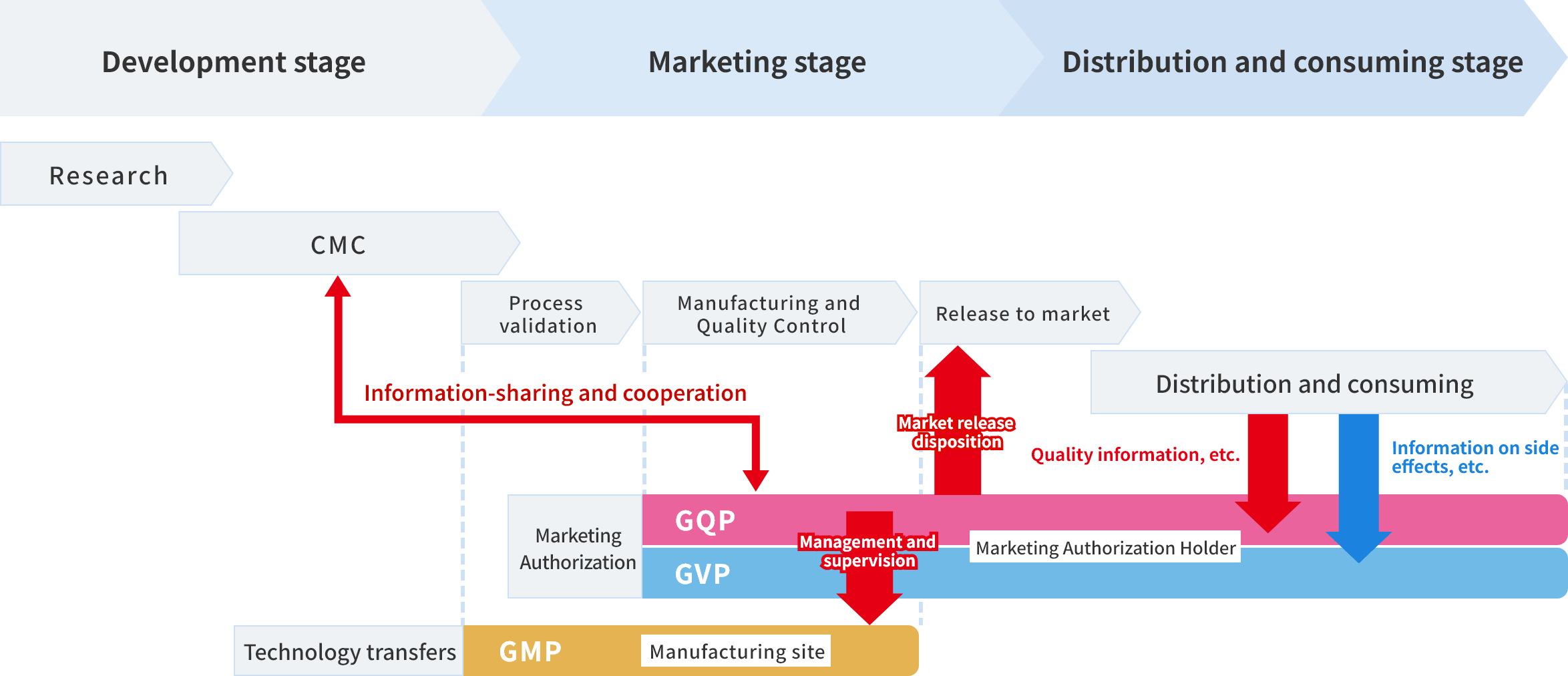
Organization and personnel for Quality Assurance duties and their roles
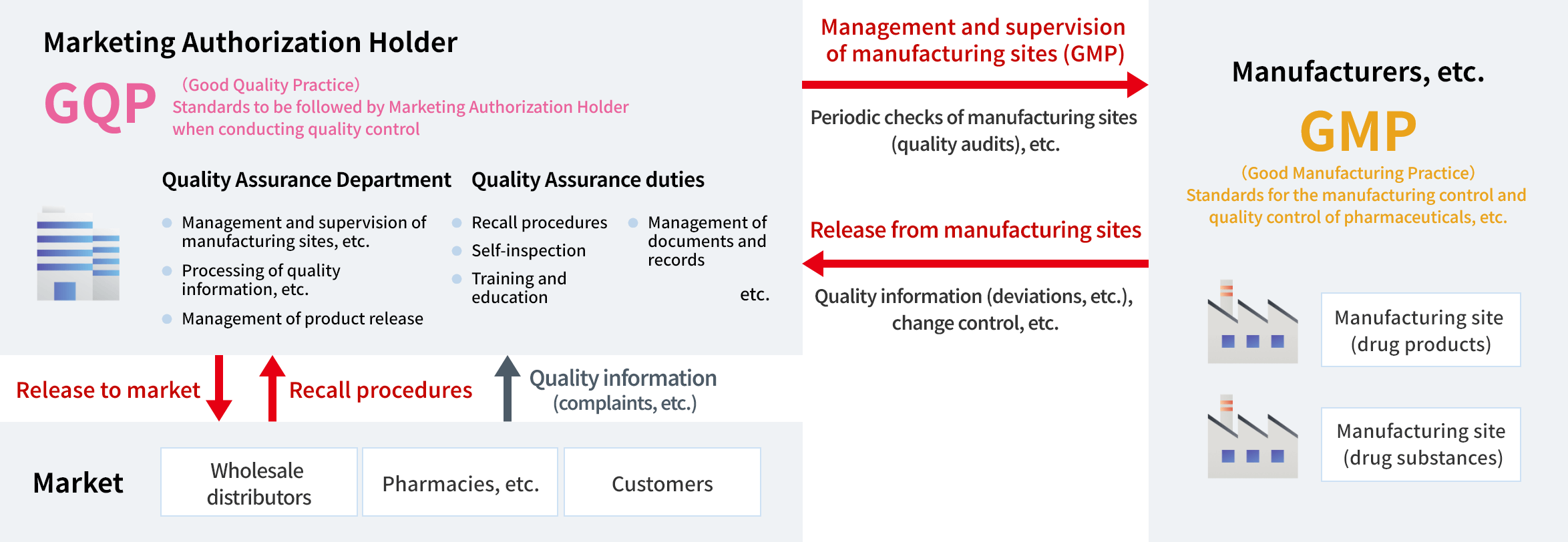
Alinamin Pharmaceutical has established the corporate philosophy “Innovating GENKI for Tomorrow.” Our Quality Assurance Department is working to fulfill this philosophy by “continuing to provide products of reliable quality to our customers.” In concrete terms, during the process of bringing a product to market, or the so-called new product development stage, we provide the development department (CMC*1) with the information necessary to ensure design quality, based on an understanding of the development policy for the new product, and we also provide support to ensure design quality at the time of technology transfer to the manufacturing sites.
After the product is launched, we monitor to ensure that reliable quality and efficacy are maintained, and we work with our group manufacturing companies and contract manufacturers to make the necessary improvements to maintain and improve our product quality.
When realizing these initiatives, we carry out our daily work in compliance with laws and regulations such as the Act on Pharmaceuticals and Medical Devices (PMD Act), GQP Ministerial Ordinance,*2 and GMP Ministerial Ordinance.*3
- *1 CMC (Chemistry, Manufacturing and Control): A series of processes related to drug formulation research, development, and product quality.
- *2 GQP (Good Quality Practice) Ministerial Ordinance: Standards that stipulate methods of quality management for drugs, quasi-drugs, cosmetics and regenerative medicine products, etc. (Ordinance of the Ministry of Health, Labour and Welfare)
- *3 GMP (Good Manufacturing Practice) Ministerial Ordinance: Standards for manufacturing control and quality control at manufacturing sites (Ordinance of the Ministry of Health, Labour and Welfare)
Product Safety
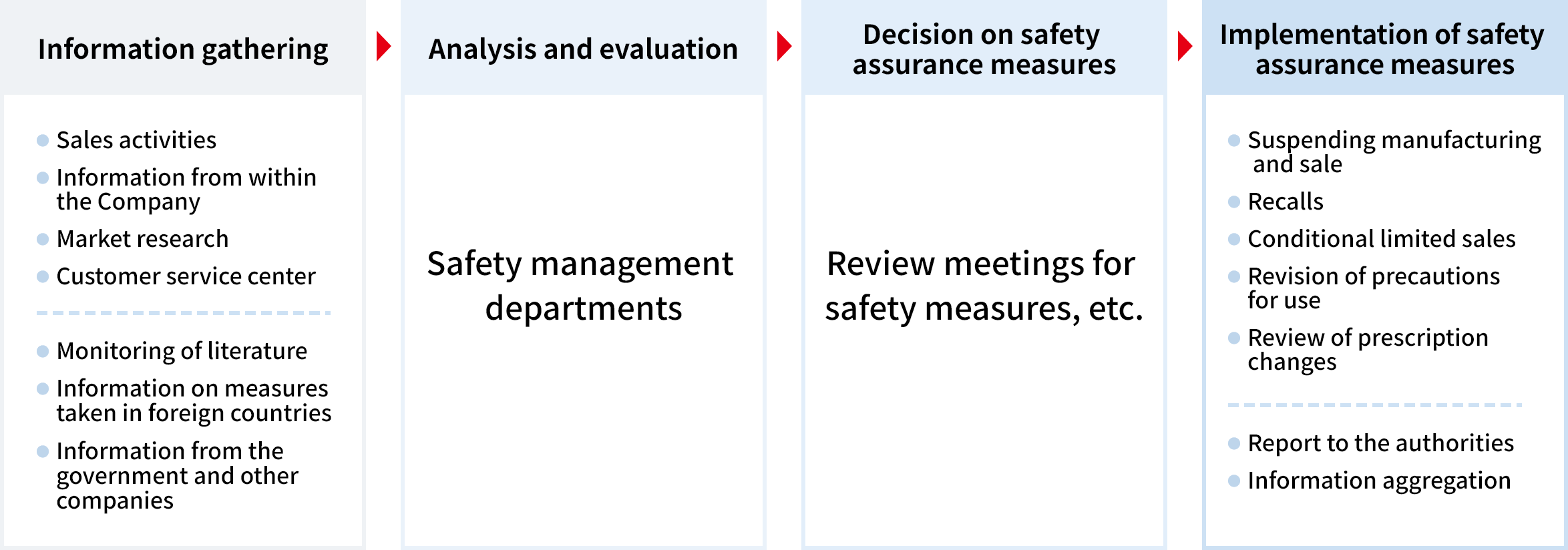
In order for our customers to be able to use our products correctly and safely, we not only ensure the safety of our products, but also carry out pharmacovigilance activities in compliance with regulations such as the Act on Pharmaceuticals and Medical Devices (PMD Act), the PMD Act Enforcement Regulations, and the GVP Ministerial Ordinance.*4
- *4 GVP (Good Vigilance Practice) Ministerial Ordinance: Standards for post-marketing safety control of pharmaceutical drugs, quasi-drugs, cosmetics, medical devices and regenerative medicine products (Ordinance of the Ministry of Health, Labour and Welfare)
In concrete terms, we collect and review safety information from pharmaceutical personnel and customers in Japan and overseas, and other information from academic conferences and literatures, and we evaluate the safety of our products while also reviewing the measures necessary to ensure safety. If it is judged that a safety warning or usage instruction is necessary, the precautions section of the package insert will be revised, and the details of the revision will be communicated to pharmacies and drugstores. In addition, we have distributed portable reference cards to all employees so that they can respond quickly and appropriately when they receive unfavorable product information about product quality or safety.

Portable reference cards
The portable reference cards include information on how to report unfavorable product information via email and provide a list of the over 10 items of information employees should gather, including the awareness date, the route of receiving the information, the product name, and more.
Product labeling and advertising activities in accordance with laws and regulations
In order to enable our customers to use our products correctly, we determine the content of the information on our product packaging and advertisements in compliance with the Act on Pharmaceuticals and Medical Devices (PMD Act), the Act against Unjustifiable Premiums and Misleading Representations, the Health Promotion Act, the Food Labeling Act, and other laws and regulations.
The information that must be shown on product packaging is stipulated in the PMD Act, etc. In the case of small-capacity product packaging and drink products, etc., the display space is limited, which sometimes makes it necessary to use small font sizes. In order to improve the readability, visibility and legibility for customers, we use universal design fonts on product labels.
When creating advertisements, we comply with legal regulations such as the PMD Act and the Act against Unjustifiable Premiums and Misleading Representations, as well as industry self-regulatory standards. We also make sure that our advertising and promotional content is fair and that it clearly conveys our company's initiatives and the value of our products to our customers.
The Japan Federation of Self-Medication Industries, of which we are a member, has established the Advertising Review Board with an aim of promoting the proper use of advertising, and conducts post-review of advertisements for pharmaceuticals. We also contribute to the optimization of advertising by participating in the Advertising Review Board as a member from the industry side.
Over the past three years (January 2022 to December 2024), we have not had any cases of product labeling or advertising violations that have resulted in us receiving recommendations from regulatory authorities (the Ministry of Health, Labour and Welfare, the Consumer Affairs Agency, the competent consumer organizations, or the Japan Advertising Review Organization (JARO)).
Turning Customer Voices into Value
We have established a Customer Relations department to respond to customer inquiries, receive feedback on our products and services, and address concerns related to quality and safety. Each member of the Customer Relations team strives to respond to customer concerns and questions with sincerity and professionalism, across a wide range of products from vitamins, cold remedies, and laxatives to food items.
Number of inquiries in fiscal year 2023: 29,346
We have established a Consumer Satisfaction Improvement (CSI) Committee to facilitate the sharing of customer feedback across relevant departments. The committee identifies and discusses feedback that requires improvement and collaborates with departments across the value chain from development to supply to enhance customer satisfaction through improvements in product quality and services. Additionally, all significant customer feedback, including complaints and critical suggestions, is reported directly to our President and Executive Officers.
The Company publishes “Think With,” a monthly newsletter from the Customer Relations department, as a way to share customer feedback company-wide. By embracing the concept of Think With-thinking alongside our customers-we aim to gain valuable insights into how we can enhance our products and services.